Explore the Frontier of AMD Treatment Research: Mooud Amirkavei’s dissertation at Aalto University School of Science, defended on 2023-05-26, offers a pioneering exploration into employing hormetic heat for rejuvenating the diminished cellular clearance mechanisms in Age-related Macular Degeneration (AMD). This research underscores the potential of hormetic heat to activate stress-response pathways, including heat shock response (HSR) and autophagy, crucial for maintaining cellular homeostasis and combating oxidative stress. Through innovative experiments involving ARPE-19 cells and animal models, Amirkavei demonstrates the precise control needed for heat shock protein expression and autophagy activation, marking a significant step towards developing non-invasive treatments for retinal degenerative diseases. This comprehensive study not only broadens our understanding of AMD’s cellular dynamics but also opens new avenues for therapeutic interventions.
https://urn.fi/URN:ISBN:978-952-64-1217-7
Abstract:
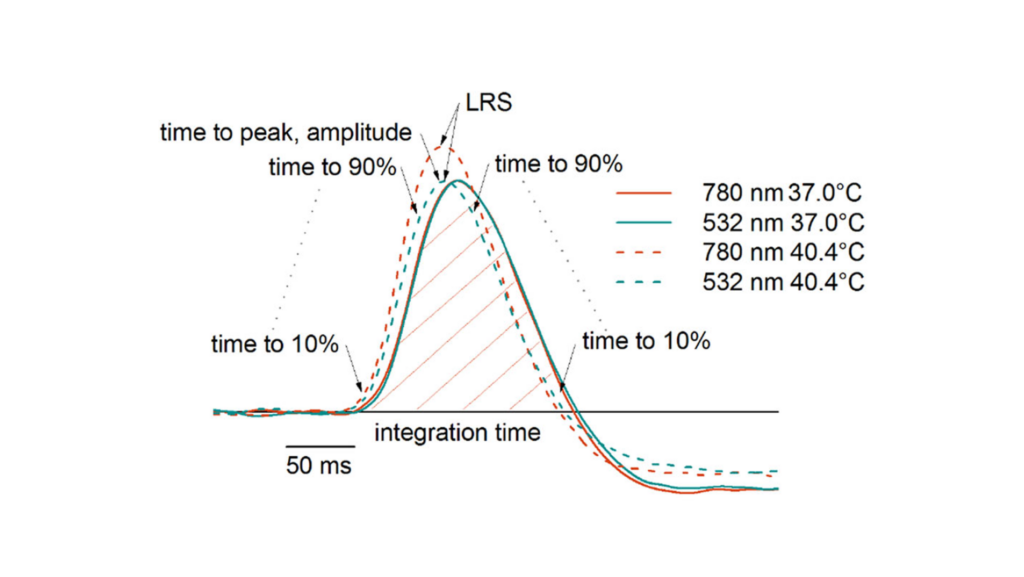
Age-related macular degeneration (AMD) is associated with decreased efficacy of cells to combat sustained oxidative stress and maintain cellular homeostasis. To cope with cellular stressors, cells have evolved stress-response pathways such as a heat shock response (HSR), which facilitate refolding of misfolded proteins and autophagy process, which facilitates lysosomal degradation and recycles cytoplasmic protein aggregates. The current research aims to assess the possibilities of employing hormetic heat to uplift the reduced activity of the senescent cellular clearance mechanisms, such as protective chaperones and autophagy, in retinal pigment epithelium (RPE) cells. In Paper I of this thesis, the human ARPE-19 cells were treated with hormetic heat shock (HHS) and the profile of autophagy expression was studied. As demonstrated by the obtained results, HHS boosts the expression of essential autophagy-associated genes in ARPE-19 cells through HSF1 activation. As a result of HHS, autophagy is activated as evidenced by a transient increase in p62/SQSTM1 and LC3B-II levels. Thus, these results highlight a role for autophagic HSF1-regulated functions and demonstrate novel evidence that autophagy contributes to hormesis in the HSR by enhancing proteostasis. In Paper II, a transpupillary 1064 nm laser heating device was developed and applied to the mouse (C57BL/6J) fundus to assess HSR. The intensity and pattern of heat shock protein 70 (HSP70) expression and the concomitant damage probability were evaluated in the mouse RPE 24 hours after laser irradiation as a function of laser power. The work demonstrated that the range of heating intensity required to produce a HSR and prevent damage to the mouse RPE is narrow. HSP70 immunostaining at 64 and 70 mW indicated a 90 and 100% probability for reliably enhanced HSP expression, respectively, whereas the equivalent probability for damage is 20 and 33 %. The study in Paper II emphasized the necessity of a retinal temperature monitoring method for controlling fundus heating treatments of retinal diseases. In Paper III, a subthreshold laser treatment (SLT) modality where the retinal temperature is monitored with electroretinography (ERG)-based thermal dosimetry in anesthetized pigs was evaluated. The ED50 peak temperature for visible lesion formation in 60-second treatments with an 810-nm laser was 48°C, and the relative temperature determination error from temperature rise was less than 10%. At 44.2 °C, HSP expression and autophagy were activated, but neither oxidative stress nor apoptosis was observed. The presented technique improves controlled activation of intracellular chaperones and waste clearance in RPE cells, with a defined temperature margin for adverse events. These findings introduced a novel treatment modality that could be beneficial in treating retinal degenerative diseases, particularly AMD.